Carbon Isotopes as Indicators of CO2 Sources (hopefully simplifyed)

One of the arguments made by global-warming deniers is that, yes, maybe atmospheric CO2 concentrations are rising, and yes, maybe those concentrations are contributing to warming of the planet, but there are many other possible sources of the additional CO2 and therefore there is much uncertainty about how much burning of fossil fuels (coal, oil, natural gas) has contributed to the increases. Not true. Scientists have, in fact, developed a means to discriminate CO2 sources using carbon isotopes ...
It is called the Suess effect. From Wikipedia: "The Suess effect is a change in the ratio of the atmospheric concentrations of heavy isotopes of carbon (13C and 14C) by the admixture of large amounts of fossil-fuel derived CO2, which is depleted in 13CO2 and contains no 14CO2. It is named for the Austrian chemist Hans Suess, who noted the influence of this effect on the accuracy of radiocarbon dating. More recently, the Suess effect has been used in studies of climate change. The term originally referred only to dilution of atmospheric 14CO2. The concept was later extended to dilution of 13CO2 and to other reservoirs of carbon such as the oceans and soils."
There are three naturally occurring isotopes of carbon: 14C, 13C, and 12C. One of these is radioactive (radiocarbon or carbon-14) and will decay over time while 13C and 12C are stable over time. The relative concentrations of these isotopes in atmospheric samples can provide a window into how much fossil fuel use has contributed to CO2 levels since the dawning of the industrial age (late 1700's).
14C
Because of it's radioactive characteristics, carbon-14 is used extensively to date organically-derived material. Again from Wikipedia: "During its life, a plant or animal is in equilibrium with its surroundings by exchanging carbon either with the atmosphere, or through its diet. It will therefore have the same proportion of 14C as the atmosphere, or in the case of marine animals or plants, with the ocean. Once it dies, it ceases to acquire 14C, but the 14C within its biological material at that time will continue to decay, and so the ratio of 14C to 12C in its remains will gradually decrease. Because 14C decays at a known rate, the proportion of radiocarbon can be used to determine how long it has been since a given sample stopped exchanging carbon – the older the sample, the less 14C will be left."
Since 14C is created at a fairly constant rate through interaction of cosmic rays with nitrogen in the atmosphere, the ratio of 14C in artifacts containing organic material to normal atmospheric concentrations (variations of which over time, such those caused by nuclear weapons testing done in the 1950's, must be accounted for) can be used to date those artifacts. Given the half-life of 14C (~5730 years) accurate measurements are limited to organic material that is less than ~50,000 years old because, beyond that, remaining concentrations of 14C are too small to accurately measure.
Fossil fuels are termed "fossil" because they were created by pressure and heat applied to organic (mostly plant) material buried millions of years ago. Therefore, given it's relatively short half-life, the concentration of 14C in fossil fuels is essentially zero. In contrast, all other significant sources of CO2 (oceanic, terrestrial, biological) contain 14C concentrations comparable to atmospheric levels. Knowing this, it can be assumed that any significant CO2 contributions to the atmosphere derived from burning fossil fuels would serve to dilute the atmospheric concentration of 14C, whereas CO2 contributions from mainly naturally occurring sources would not dilute atmospheric 14C levels to any significant extent.
Given this dichotomy, by measuring 14C concentrations (14C/12C) in atmospheric samples over time, 14C can be used as an "isotopic fingerprint" to identify the level of fossil fuel contributions to overall atmospheric CO2. The following comparison is indicative of the contributions of fossil fuel CO2 to the atmosphere since the beginning of the industrial revolution:
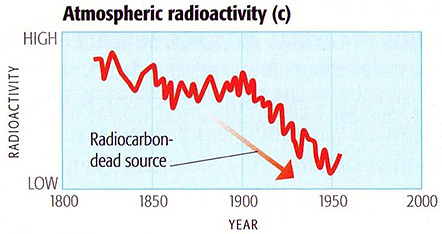
This comparison cannot be extended beyond 150 years because of above-ground atomic bomb testing that occurred during the late 1940s and early 1950s (which added large amounts of 14C to the atmosphere). However, sampling over shorter periods of time since the 1950s indicates a steady drop in 14C concentrations as atmospheric CO2 levels continued to rise:

Samples were taken by NOAA at a site at 11,000 feet elevation in Colorado where air is well-mixed and remote from fossil-fuel sources and so is considered to be representative of worldwide atmospheric conditions. The steady drop in 14C over a period when worldwide CO2 concentrations were steadily rising is indicative of dilution by a CO2 source lacking in 14C (i.e., fossil fuels).
13C
For different reasons, the 13C carbon isotope can be used in a manner similar to 14C to assess the contribution of fossil-fuel sources to the global increases in atmospheric CO2. When plants use CO2 to perform photosynthesis, they preferentially intake 12C over the heavier 13C isotope; and in the course of synthesizing CO2 into organic matter (e.g., sugars, wood) they once again prefer 12C over the heavier 13C. The result of this process is that the CO2 captured and stored in plants contains a lower ratio of 13C/12C than exists in atmospheric CO2. Since fossil fuels are comprised primarily of ancient plants, this lower ratio is a characteristic of CO2 derived from burning of fossil fuels. The more fossil fuels contribute atmospheric CO2 the lower the 13C/12C ratio becomes.
The following chart relates changes in atmospheric CO2 concentrations to changes in the atmospheric carbon isotope 13C concentrations over time.

The squiggly nature of the plots reflects seasonal changes in CO2 - in summer, the plants pull CO2 out of the atmosphere; in winter, photosynthesis stops and some of the stored CO2 is returned to the atmosphere through respiration. As a result, in the summer, more of the 12C than the 13C isotope is preferentially extracted from the atmosphere (13C/12C ratio increases); in the winter, more 12C than 13C is released to the atmosphere (13C/12C ratio decreases). The overall 13C/12C trend, however, is downward indicating fossil fuel contributions to the upward trending CO2 levels.
The image below shows the same CO2 v. 13C comparison over a longer period of time as derived from ice core samples taken in Antarctica.

One thing that strikes me about this chart is that although other sources of CO2 such as plant burning, decomposition, and respiration, oceanic release, and geologic activity such as vulcanism (often cited by deniers*) were active throughout the whole time period between 1000 and 1800 AD, CO2 levels remained relatively stable. It was not until the start of the industrial revolution (driven by fossil fuels) in the late 1700s that CO2 levels began to rapidly rise in negative covariance with the fossil fuel isotopic fingerprints.
Although I am a geologist, I do not claim to be an expert on climate change or isotopic analysis, but my academic and experiential background coupled with the studying I have done to date has provided me with no doubt that climate change is the existential issue of our time. I wrote this blog entry in part to learn more about how climate scientists use carbon isotopes to determine the origin of the rapidly increasing CO2 in our atmosphere. If you want to read in more depth from the real experts, here are some excellent references:
- NOAA Earth System Research Laboratory - The Basics: Isotopic Fingerprints
- A revised 1000 year atmospheric δ13C‐CO2 record from Law Dome and South Pole, Antarctica
- Volcanic Versus Anthropogenic Carbon Dioxide
- Pre-industrial anthropogenic CO2 emissions: How large?
- Which emits more carbon dioxide: volcanoes or human activities?
- How do we know that recent CO2 increases are due to human activities?
- The Discovery of Global Warming
* From the US Geological Survey: "Carbon dioxide (CO2) is a greenhouse gas and is the primary gas blamed for climate change. While sulfur dioxide released in contemporary volcanic eruptions has occasionally caused detectable global cooling of the lower atmosphere, the carbon dioxide released in contemporary volcanic eruptions has never caused detectable global warming of the atmosphere. In 2010, human activities were responsible for a projected 35 billion metric tons (gigatons) of CO2 emissions. All studies to date of global volcanic carbon dioxide emissions indicate that present-day subaerial and submarine volcanoes release less than a percent of the carbon dioxide released currently by human activities. While it has been proposed that intense volcanic release of carbon dioxide in the deep geologic past did cause global warming, and possibly some mass extinctions, this is a topic of scientific debate at present." "There continues to be efforts to reduce uncertainties and improve estimates of present-day global volcanic CO2 emissions, but there is little doubt among volcanic gas scientists that the anthropogenic CO2 emissions dwarf global volcanic CO2 emissions."